Optimum Materials for Storing Heat
If you don’t want you’re stove to go cold immediately after the fire is out, you need to store some of the heat from combustion. A wood-burning heater's ability to capture and store excess heat for gradual release later, to a large extent, is governed by the materials used. There are several important physical properties that govern a material’s ability to absorb the intense excess heat from burning wood.
Heat Storage Capacity
In the table below, there are a number of candidate materials. The most obvious characteristic to be considered is the ability to store as much heat as possible in a given amount of space. This ability is called heat capacity, and the table below is sorted by heat capacity.
Heat capacity is calculated by multiplying the density of a material by its ability to store heat. The later is referred to as its specific heat. As you see from the chart below, you need both high specific heat and high density to have high heat capacity. Lots of materials are dense but do not hold heat well, or hold heat well, but are not very dense.
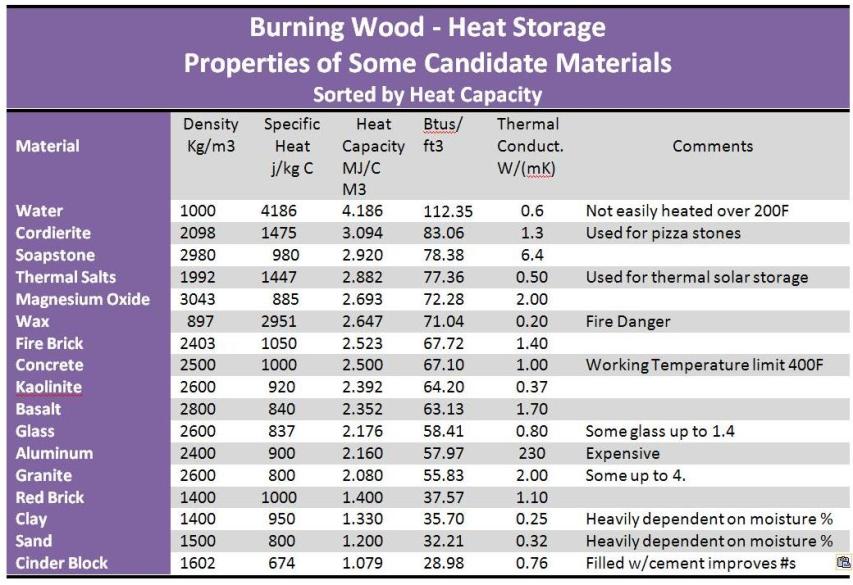
Working Temperature
Heat Capacity is a rating per degree that the material’s temperature is raised. The key to dense energy storage is to be able to raise the material the maximum amount possible with the fire’s exhaust. For example, comparing water to red brick, we find that water has a much higher heat capacity. However, since water turns to vapor so quickly, it can’t absorb very many degrees of heat relative to a red brick. So while water is an excellent heat transport material, it is probably not as good of a choice as another material which can be raised 500°.
Concrete also has good heat capacity, but it has a working temperature limit of about 400°F. Overshooting this number will cause stove failure. Any time we utilize CMUs (made from concrete) in a design, we use a layer of fireclay bricks between the CMUs and the heat to buffer the higher temperatures.
Glass takes about 2700°F to melt it. On the other hand, aluminum melts at 1220°F.
Thermal salts and magnesium oxide must be contained; at temperature, they are liquid, complicating construction.
Thermal Conductivity
Thermal Conductivity is a measure of the material’s ability to absorb heat. You can see from the chart that the high(ish) thermal conductivity of soapstone along with its high working temperature and heat capacity make it an attractive material for wood burning stove. It is more expensive than fire brick, but looks much better.
Likewise clay is very cheap, but is many times less conductive than fire clay brick.
Form Limitations
Cordierite is available as kiln “furniture”, such as round and rectangular shelves in addition to pizza stones. We were unable to find a way to cast a shape of our choice, so you would have to build your design to incorporate a shelf of a specific dimension.
Chart Numbers
Testing methods, samples used for testing, the type of material, all conspire to create a wide variety of specifications available for any given material. Also very few sources use the same unit of measure. So when comparing materials be sure to do the conversions. The numbers shown in the chart above are only intended to provide a ball park starting place.
There are no products listed under this category.
